A research team led by Researcher Tan Hairen of Nanjing University published a research paper titled "Scalable fabrication of wide-bandgap perovskites using green solvents for tandem solar cells" in the journal Nature Energy. Team member Duan Chenyang is the first author, and Researcher Tan Hairen is the corresponding author.
Key Highlights: This paper proposes a green solvent system consisting of dimethyl sulfoxide and acetonitrile to effectively dissolve cesium and bromide salts. The addition of ethanol prevents precursor degradation and extends the solution processing window. Wide-bandgap solar cells fabricated by the doctor blade coating method achieved power conversion efficiencies of 19.6% (at 1.78 eV) and 21.5% (at 1.68 eV), respectively. Furthermore, a 20.25 cm² all-perovskite tandem solar module achieved a power conversion efficiency of 23.8%.
The commercialization of perovskite-based tandems requires environmentally friendly solvents for the scalable fabrication of efficient wide-bandgap (WBG) perovskites (1.65-1.80 eV). However, due to the low solubility of cesium and bromide salts, WBG perovskites rely on the toxic N,N-dimethylformamide solvent. Therefore, green solvents developed for formamidine-type lead iodide perovskites with a bandgap of ~1.50 eV are unsuitable for WBG perovskites. To address this issue, the team led by Researcher Tan Hairen of Nanjing University proposed an efficient and environmentally friendly green solvent system, using a mixture of DMSO, acetonitrile, and ethanol to dissolve cesium and bromide salts. This system prevents precursor degradation and extends the processing window, allowing for the formation of dense and void-free perovskite films over large areas, thereby increasing the scalability of WBG perovskite fabrication. Using this green solvent mixture, blade-coated WBG perovskite solar cells achieved power conversion efficiencies of 19.6% (at 1.78 eV) and 21.5% (at 1.68 eV), respectively. PCEs of 26.3% and 27.8% were achieved in 1 cm² all-perovskite and perovskite/silicon tandem solar cells, respectively. An all-perovskite tandem solar module (with an aperture area of 20.25 cm²) achieved a PCE of 23.8%. Furthermore, the green solvent system also enabled the fabrication of WBG PSCs in ambient air with negligible PCE degradation, achieving a PCE of 23.1% for a 20.25 cm² all-perovskite tandem module. Finally, the green solvent system was demonstrated to be suitable for NBG PSC fabrication, enabling the production of a 20.25 cm² all-perovskite tandem module with a PCE of 22.2% for both WBG and NBG subcells using only green solvents.
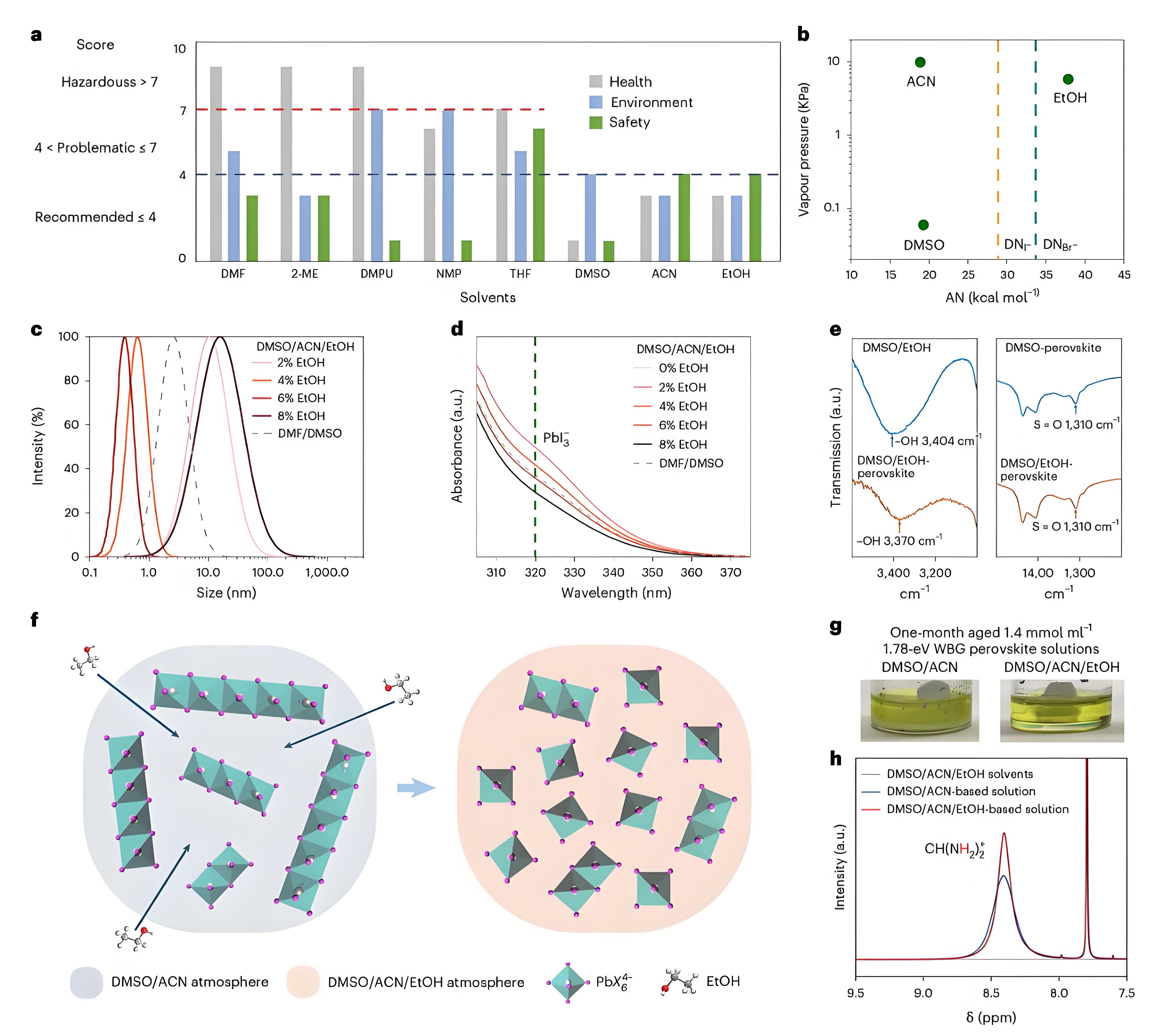
Fig. 1 | Solvents and colloid properties of the EtOH-incorporated perovskite precursor solution. a, Safety, health and environment impact of solvents outlined by CHEM21. b, Vapour pressure and AN of the three studied solvents and DN of iodide and bromide ions. c, Colloidal size distribution of WBG perovskite precursor solution in multiple solvent systems. d, UV–vis absorption spectra of PbI2 dissolved in DMF/DMSO and in DMSO/ACN with various volumes of EtOH. e, FTIR spectra of DMSO/EtOH solvent, perovskite in DMSO/EtOH solution, perovskite in DMSO solution. f, Schematic illustration of the colloidal components after adding EtOH into the perovskite precursor solution. g, Pictures of one-month-aged 1.78 eV WBG precursors dissolved in DMSO/ACN and DMSO/ACN/EtOH solvent systems. h, 1H NMR spectra of only solvents [DMSO-d6/ACN-d3/EtOH], DMSO-d6/ACN-d3-based perovskite solution and DMSO-d6/ACN-d3/EtOHbased perovskite solution. The H corresponding to the position is marked in red. δ is the chemicalshift.
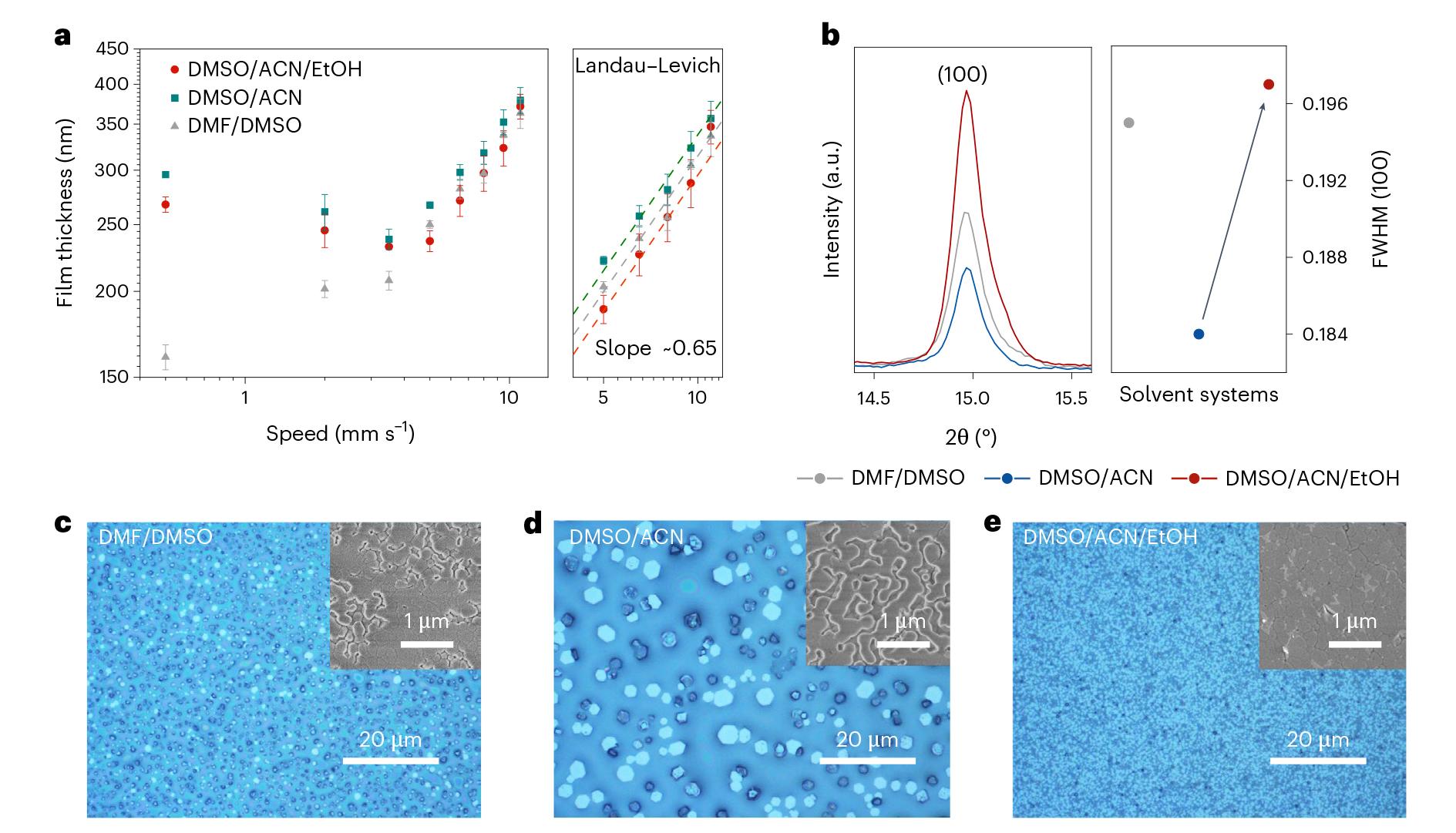
Fig. 2 | Crystallization kinetics of blade-coated perovskite films. a, log(v) − log(t) plot of perovskite film deposition from three different solvent systems. Each error bar is statistically derived from three sets of experimental data. The dashed lines represent the fitted lines of each solvent systems. b, XRD patterns and FWHM of wet perovskite films fabricated by blade coating at 9.5 mm s−1 with 40 kPa gas blowing followed by natural drying for 180 s. The arrow shows the change of FWHM after EtOH existence. c–e, Microscopy photographs of the corresponding perovskite films as described in b and top-view scanning electron microscope (SEM) images (insets) of WBG perovskite films peeled off from indium tin oxide (ITO) glass substrate to evaluate perovskite–substrate interface.
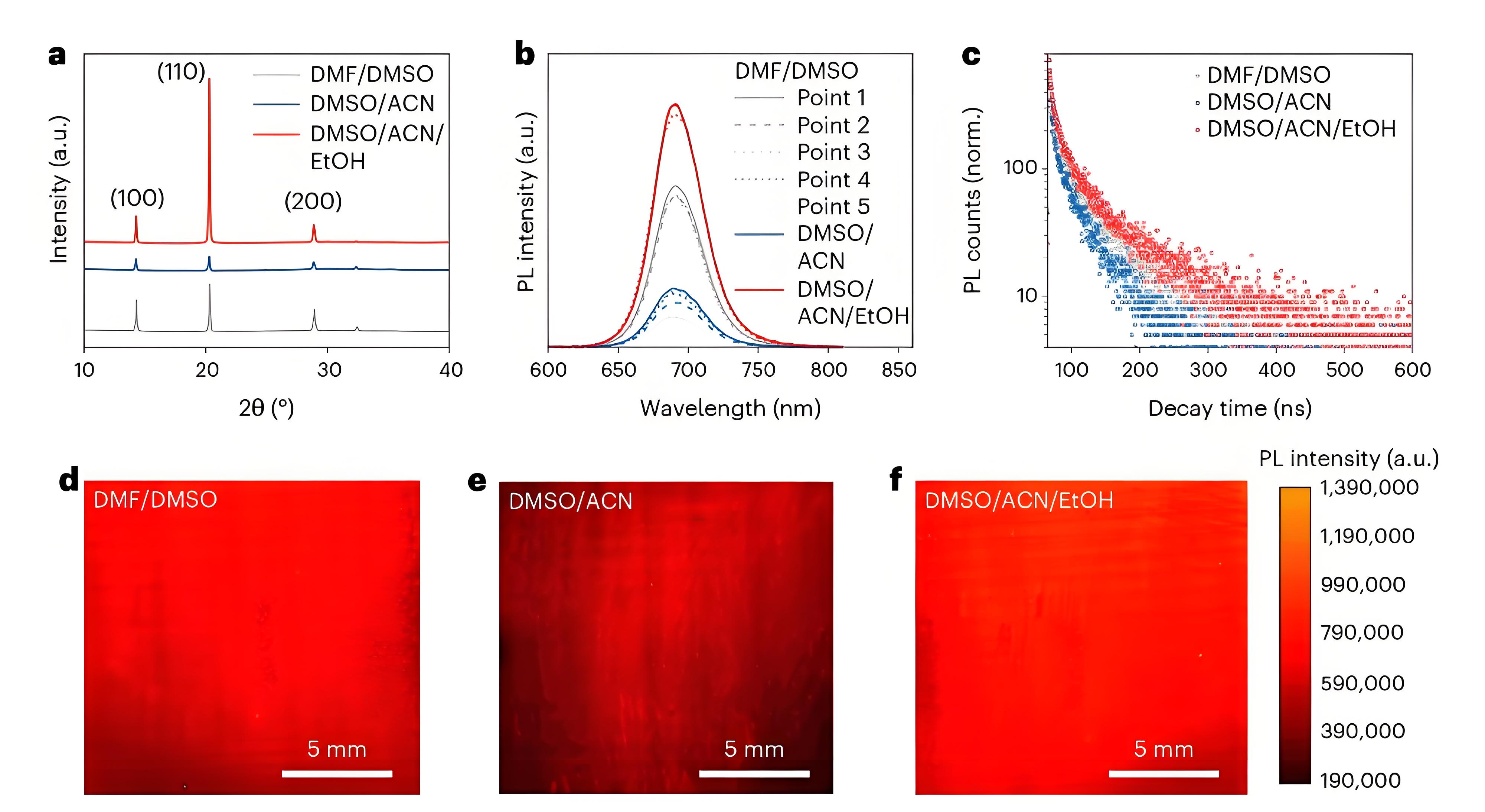
Fig. 3 | Characterization of WBG perovskite films fabricated utilizing the three solvent systems. a, XRD patterns of FA0.65Cs0.35PbI1.8Br1.2 perovskite films. b,c, PL and time-resolved photoluminescence spectra excited from the perovskite side of blade-coated WBG perovskite films. PL counts (norm.) indicates normalized PL counts. d–f, PL mapping images of a 1.5-cm-by-1.5-cm area of blade-coated WBG perovskite films.
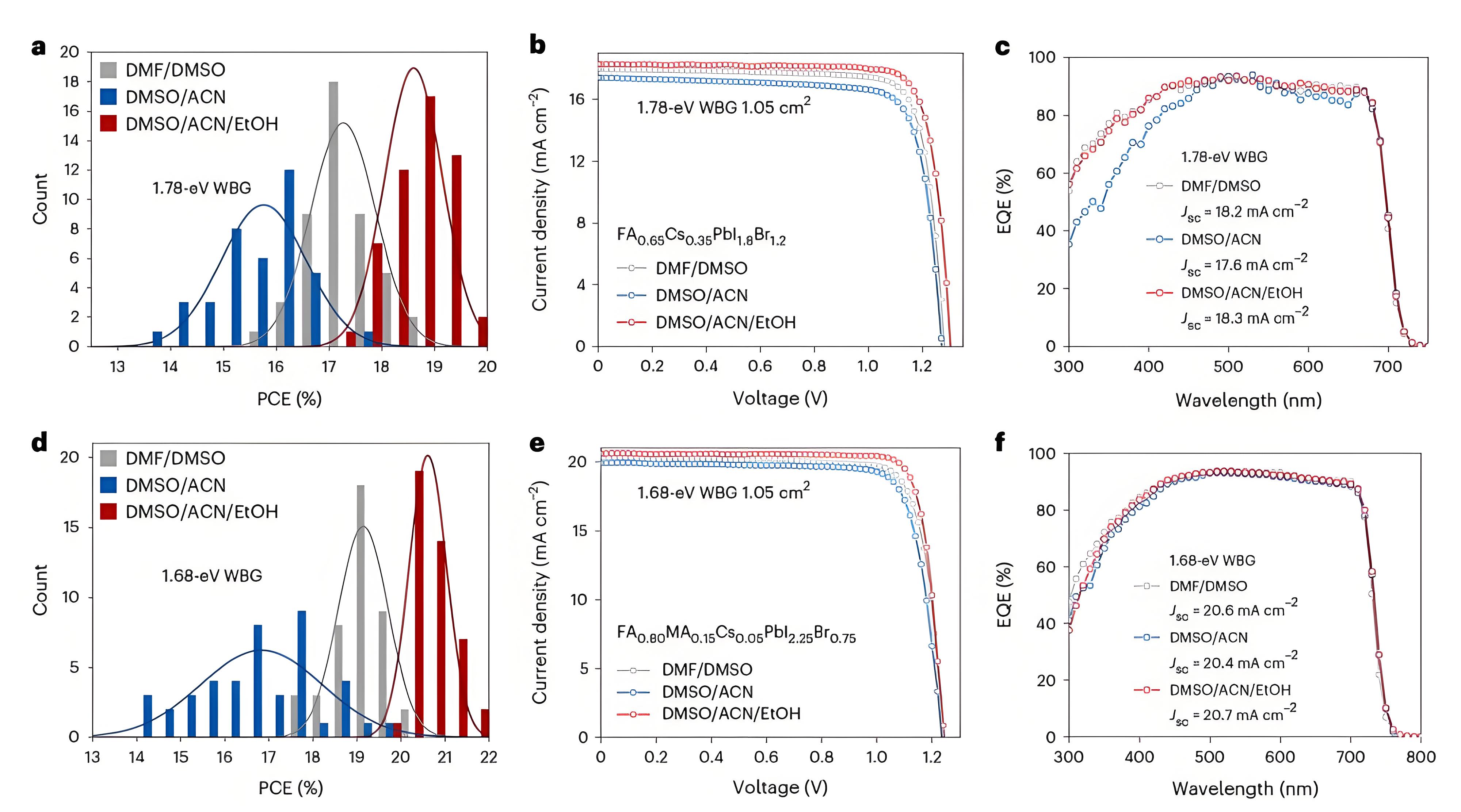
Fig. 4 | Photovoltaic performance of 1.78 eV and 1.68 eV WBG PSCs prepared utilizing different solvent systems. a–c, Comparison of PCE (a), J–V curves (b) and EQE spectra (c) of 1.78 eV bandgap perovskite devices. d–f, Comparison of PCE (d), J–V curves (e) and EQE spectra (f) of 1.68 eV bandgap perovskite devices. The fitted curve from a and d follows a Gaussian distribution.

Fig. 5 | Photovoltaic performance of all-perovskite tandem solar cells and large-area perovskite tandem modules. a, Histogram of PCEs for 30 allperovskite tandem devices and a picture of 1-cm2 monolithic all-perovskite tandem solar cells. The fitted curve follows a Gaussian distribution. b, J–V curves of the champion all-perovskite tandem solar cells based on 1.78-eV WBG subcells (aperture area of 1.05 cm2). c,d, J–V (c) and EQE (d) curves of the champion all-perovskite tandem module (aperture area of 20.25 cm2, eight subcells in series). e, J–V curves of champion all-perovskite tandem module with WBG fabricated in ambient air (30% RH, aperture area of 20.25 cm2, eight subcells in series). f, J–V curves of all green solvent-based champion all-perovskite tandem module (aperture area of 20.25 cm2, eight subcells in series).
本文来源:DOI: 10.1038/s41560-024-01672-x
https://doi.org/10.1038/s41560-024-01672-x